Genetic Testing & Why
Breeding for Genetic Health
By and large, we are very fortunate that Scottish Collies are pretty healthy dogs. However, there are a few inherited diseases that have been identified as being a problem for the various Collie breeds. It is the goal of the Scottish Collie Preservation Society to acknowledge that these harmful diseases exist; to educate our members, breeders, and the public about them; and to provide SCPS breeders with the tools and information that will ultimately enable our community to breed healthier dogs.
Scottish Collie vs Rough Collie in Regards to Genetic Diversity
For the past year, UC Davis and Betterbred have been studying the Genetic health and diversity of the Scottish Collie vs. the Rough Collie. The initial results have indicated the the Scottish Collie is more genetically diverse than the purebred rough collies in closed registries. This is very important as genetic diversity is linked to larger, healthier litters, stronger immunity systems and overall better health for a breed. SCPS will continue to work with Betterbred and UC Davis to breed towards genetic diversity.
“A standard genetic assessment was made on 55 conformation Collie for the purpose of breed comparison The Scottish Collie were more genetically diverse than Collie (Na = 6.12 vs 5.33) and had higher observed heterozygosity (Ho = 0.59 vs. 0.44). The average effective alleles per locus was higher (Ne = 2.76) in Scottish Collie than Collie (Ne = 2.12). The Scottish Collie were 2.2% more heterozygous (more outbred) than expected for a random breeding population (F = -0.022), while the Collie were 9% less heterozygous (more inbred) than expected (F = 0.09).”
The Scottish Collie and Rough Collie were starting to differentiate into genetically distinct populations (i.e., subpopulations) – more like North American and European Italian Greyhounds or performance and conformation types of Golden Retrievers, but not yet to the level of varieties, such as seen with American and Japanese Akita, or Black and Pepper and Salt Giant Schnauzers. The Border Collies were genetically distinct from the Scottish Collie and Collie and manifested a surprising degree of genetic diversity between individuals, similar to Labrador and Golden Retrievers.
The decision will remain with the Scottish Collie Preservation Society and other such efforts to decide what to do with their data and whether the existing biodiversity they have secured from the remnants of the original Scottish Collie is sufficient. In the meantime, BetterBred’s breed management tools will help them effectively maintain this biodiversity. This can be accomplished by breeding for dogs that are less related to other dogs in the database, dogs that have genetics that are not well represented in the population as well as selecting for litters whose parents will be as unrelated as is reasonable (and therefore reducing inbreeding in offspring).
A striking observation was the comparison of the Scottish Collies with that of the mainstream show Collies – this comparison showed that the Collie today has much less biodiversity than the Scottish Collie, as well as much less biodiversity in the DLA. It will be interesting to see how this trend develops as more Collies are tested.
Dr. Niels Pedersen 2019
UC Davis has been releasing preliminary breed reports once they feel that they have enough dogs to take a very initial look at a breed. Depending on the sample and how well those initial dogs were selected, it is possible they will represent a good snapshot of the breed. Of course, results will be as unrelated as is reasonable (and therefore reducing inbreeding in offspring).
Genetics Primer in 156 Words
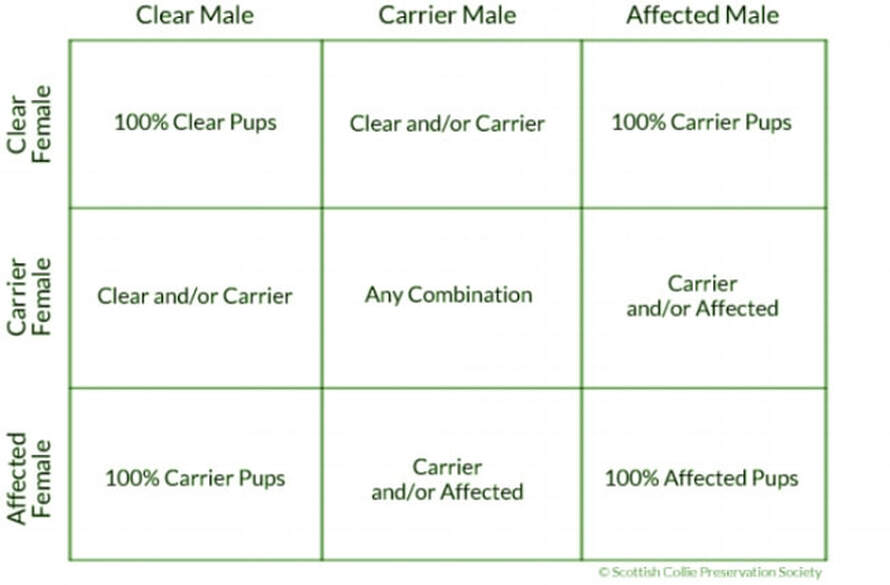
Each dog receives a specific matched set of genes at the moment of their conception: half from their dam, and half from their sire. These genes define everything, including temperament, color, working ability, health, and 10,000 other things. Many diseases are known as "autosomal recessive" diseases, meaning simply that a dog must inherit one copy of the gene mutation from each parent for the disease to be present in the pup; these pups are then "Affected". If they receive one copy of the mutation from only one parent, the dog is what's known as a "Carrier" for that disease, meaning that he won't develop the disease himself, but can pass it on to his pups. If they don't receive a copy of the mutation from either parent, they are "Clear" and will never develop that particular disease. Below is a table that explains what to expect from puppies depending on the disease status of each parent.
By and large, we are very fortunate that Scottish Collies are pretty healthy dogs. However, there are a few inherited diseases that have been identified as being a problem for the various Collie breeds. It is the goal of the Scottish Collie Preservation Society to acknowledge that these harmful diseases exist; to educate our members, breeders, and the public about them; and to provide SCPS breeders with the tools and information that will ultimately enable our community to breed healthier dogs.
Scottish Collie vs Rough Collie in Regards to Genetic Diversity
For the past year, UC Davis and Betterbred have been studying the Genetic health and diversity of the Scottish Collie vs. the Rough Collie. The initial results have indicated the the Scottish Collie is more genetically diverse than the purebred rough collies in closed registries. This is very important as genetic diversity is linked to larger, healthier litters, stronger immunity systems and overall better health for a breed. SCPS will continue to work with Betterbred and UC Davis to breed towards genetic diversity.
“A standard genetic assessment was made on 55 conformation Collie for the purpose of breed comparison The Scottish Collie were more genetically diverse than Collie (Na = 6.12 vs 5.33) and had higher observed heterozygosity (Ho = 0.59 vs. 0.44). The average effective alleles per locus was higher (Ne = 2.76) in Scottish Collie than Collie (Ne = 2.12). The Scottish Collie were 2.2% more heterozygous (more outbred) than expected for a random breeding population (F = -0.022), while the Collie were 9% less heterozygous (more inbred) than expected (F = 0.09).”
The Scottish Collie and Rough Collie were starting to differentiate into genetically distinct populations (i.e., subpopulations) – more like North American and European Italian Greyhounds or performance and conformation types of Golden Retrievers, but not yet to the level of varieties, such as seen with American and Japanese Akita, or Black and Pepper and Salt Giant Schnauzers. The Border Collies were genetically distinct from the Scottish Collie and Collie and manifested a surprising degree of genetic diversity between individuals, similar to Labrador and Golden Retrievers.
The decision will remain with the Scottish Collie Preservation Society and other such efforts to decide what to do with their data and whether the existing biodiversity they have secured from the remnants of the original Scottish Collie is sufficient. In the meantime, BetterBred’s breed management tools will help them effectively maintain this biodiversity. This can be accomplished by breeding for dogs that are less related to other dogs in the database, dogs that have genetics that are not well represented in the population as well as selecting for litters whose parents will be as unrelated as is reasonable (and therefore reducing inbreeding in offspring).
A striking observation was the comparison of the Scottish Collies with that of the mainstream show Collies – this comparison showed that the Collie today has much less biodiversity than the Scottish Collie, as well as much less biodiversity in the DLA. It will be interesting to see how this trend develops as more Collies are tested.
Dr. Niels Pedersen 2019
UC Davis has been releasing preliminary breed reports once they feel that they have enough dogs to take a very initial look at a breed. Depending on the sample and how well those initial dogs were selected, it is possible they will represent a good snapshot of the breed. Of course, results will be as unrelated as is reasonable (and therefore reducing inbreeding in offspring).
Genetics Primer in 156 Words
Each dog receives a specific matched set of genes at the moment of their conception: half from their dam, and half from their sire. These genes define everything, including temperament, color, working ability, health, and 10,000 other things. Many diseases are known as "autosomal recessive" diseases, meaning simply that a dog must inherit one copy of the gene mutation from each parent for the disease to be present in the pup; these pups are then "Affected". If they receive one copy of the mutation from only one parent, the dog is what's known as a "Carrier" for that disease, meaning that he won't develop the disease himself, but can pass it on to his pups. If they don't receive a copy of the mutation from either parent, they are "Clear" and will never develop that particular disease. Below is a table that explains what to expect from puppies depending on the disease status of each parent.
The wonderful thing about genetics is that once you understand the simple rules of inheritance for autosomal recessive diseases (what we're mostly concerned with in the Scottish Collie), you can then seek out dogs that will help to advance the genetic profile of your own breeding program. It takes time, determination, and a like-minded community that shares the same goal. To that end, we earnestly hope that you will come with us in the effort to preserve the Scottish Collie.
DISEASES OF PRIMARY CONCERN
[ MDR1 ] MULTI-DRUG RESISTANCE MUTATION
Multidrug resistance 1, also called MDR1, is an inherited condition affecting several breeds of dogs, especially herding dogs such as the Scottish collie. The Mutation in the ABCB1 gene associated with MDR1 causes dysfunction of P-glycoprotein, which is responsible for removing certain drugs and toxins from the body. Clinical signs are most commonly associated with distribution of the drug in the central nervous system. MDR1 is inherited in an autosomal incomplete dominant manner in dogs meaning that dogs only need to inherit one copy of the mutated gene to be at an increased risk of developing adverse reactions to certain medications. Though adverse reactions to certain drugs are most commonly seen in dogs having two copies of the mutated gene, Carrier dogs can also experience drug sensitivities and dosages need to be adjusted accordingly. Thus, dogs that have one or two mutant copies of the gene are considered at-risk for adverse drug reactions. If an at-risk dog is treated with one of several common drugs (see below*), they are at risk of developing neurologic symptoms that could range from tremors, excess salivation, anorexia, and blindness to coma and even death. Because of the defective ability to metabolize specific drugs, these drugs can be lethal even at low doses. The MDR1 mutation does not cause adverse effects in dogs unless the dog is exposed to these drugs. Therefore, veterinarians should be notified when a dog is at risk for multidrug resistance 1 prior to administration of any medications.
*Drugs known to cause neurological signs related to the MDR1 mutation:
Acepromazine, butorphanol, doxorubicin, emodepside, erythromycin, ivermectin, loperamide, milbemycin, moxidectin, rifampin, selamectin, vinblastine and vincristine.
In addition to this list, there are many other drugs known to be removed from the central nervous system via the P-glycoprotein mechanism in humans. However, reports of neurological dysfunction related to drugs other than those listed here are scarce in dogs. Please consult your veterinarian prior to giving drugs to known multidrug resistance carriers, affected dogs, or untested dogs of breeds commonly affected with this condition.
[ CEA ] COLLIE EYE ANOMALY
Collie Eye Anomaly (CEA), also known as choroidal hypoplasia (CH), is an inherited disease affecting several dog breeds including the Scottish collie. The choroid is the layer of tissue in the eye responsible for supplying blood and nutrients to the Retina. In dogs affected with CEA, the choroid does not develop properly and is therefore thinner than normal. The severity of the condition can vary from dog to dog. In mild cases, affected dogs may only show signs of collie eye anomaly on eye exam between about 5 and 12 weeks of age, just prior to normal, age-related pigmentation of the retina which often masks the characteristic, disease-related changes. After this time period, mildly affected dogs may be impossible to distinguish from normal dogs on eye exam (a phenomenon often referred to as “going normal”) and may not display obvious vision deficits. In more severely affected dogs, clinical signs include malformations of the eye and/or optic nerve (colobomas), retinal detachment, intraocular bleeding, and subsequent blindness. Both mild and severe forms of CEA are associated with the same NHEJ1gene Mutation. Therefore, predicting the potential severity of the disease in an affected puppy is difficult as mildly affected parents may produce offspring that are severely affected.
DISEASES OF SECONDARY CONCERN
[ CN ] CYCLIC NEUTROPENIA
Cyclic neutropenia is an inherited disease affecting Scottish collies. Affected dogs undergo an oscillating cycle in the quantity of a type of white blood cells called neutrophils, important for controlling and preventing bacterial and fungal infections. Affected dogs have Neutrophil counts oscillating between normal quantities to almost zero neutrophils on an approximate 2 week frequency. Affected puppies often die within a few days of birth or are stunted in growth. Affected dogs have a gray coat color and are vulnerable to infections during periods of low neutrophil counts. Symptoms associated with this condition are normally seen during or immediately after a period of low neutrophil counts. Symptoms include fever, diarrhea, inflamed lymph nodes, gingivitis, lameness and mild bleeding episodes. Even with medical care, most dogs die before 2 years of age due to liver or kidney failure.
[ DM ] DEGENERATIVE MYELOPATHY
Degenerative Myelopathy caused by Mutation of the SOD1 gene is an inherited neurologic disorder of dogs. This mutation is found in many breeds of dog, including the Scottish collie. While it is not clear for some of the other breeds, collies are known to develop degenerative myelopathy associated with this mutation. The variable presentation between breeds suggests that there are environmental or other genetic factors responsible for modifying disease expression. The average age of onset for dogs with degenerative myelopathy is approximately nine years of age. The disease affects the White Matter tissue of the spinal cord and is considered the canine equivalent to amyotrophic lateral sclerosis (Lou Gehrig’s disease) found in humans. Affected dogs usually present in adulthood with gradual muscle Atrophy and loss of coordination typically beginning in the hind limbs due to degeneration of the nerves. The condition is not typically painful for the dog, but will progress until the dog is no longer able to walk. The gait of dogs affected with degenerative myelopathy can be difficult to distinguish from the gait of dogs with hip dysplasia, arthritis of other joints of the hind limbs, or intervertebral disc disease. Late in the progression of disease, dogs may lose fecal and urinary continence and the forelimbs may be affected. Affected dogs may fully lose the ability to walk 6 months to 2 years after the onset of symptoms. Affected medium to large breed dogs, such as the rough collie, can be difficult to manage and owners often elect euthanasia when their dog can no longer support weight in the hind limbs.
[ PRA/PRCD ] PROGRESSIVE RETINAL ATROPHY / PROGRESSIVE ROD-CONE DISEASE
Genetic testing of the RD3 gene will reliably determine whether a dog is a genetic Carrier of progressive retinal Atrophy, Rod-cone dysplasia 2. Progressive retinal atrophy, rod-cone dysplasia 2 is inherited in an Autosomal Recessive manner in dogs meaning that they must receive two copies of the mutated gene (one from each parent) to develop the disease. In general, carrier dogs do not have features of the disease but when bred with another carrier of the same Mutation, there is a risk of having affected pups. Each pup that is born to this pairing has a 25% chance of inheriting the disease and a 50% chance of being a carrier of the RD3 gene mutation. Reliable genetic testing is important for determining breeding practices. In order to eliminate this mutation from breeding lines and to avoid the potential of producing affected pups, breeding of known carriers to each other is not recommended. Dogs that are not carriers of the mutation have no increased risk of having affected pups. However, because there are multiple types of PRA caused by mutations in other genes, a normal result in RD3does not exclude PRA in a pedigree.
[ VWD II ] VON WILLEBRAND'S DISEASE (TYPE II)
VWDII is an inherited blood-clotting disease. Symptoms can include easy bruising, frequent nosebleeds, lots of bleeding from teething, and excessive bleeding from surgery or trauma. When dogs are known VWD-Affected, surgeons can be ready ahead of time with extra blood units on hand, but can be caught off guard when it's not known that a patient is Affected. VWD could also be a problem for simple everyday concerns such as nail trims, but it's especially important for dogs who work bigger stock such as swine or cattle.
[ HUU ] Hyperuricosuria
Hyperuricosuria is an inherited condition of the urinary system affecting several breeds of dog. The SLC2A9 gene codes for a protein that allows the kidneys to transport uric acid from the urine. Dogs with mutations in both copies of the SLC2A9 gene are predisposed to have elevated levels of uric acid in the urine, hence the name hyperuricosuria. Uric acid can form crystals and/or stones (uroliths) in the urinary tract. Dogs with hyperuricosuria most commonly present with symptoms of recurrent urinary tract inflammation, which include frequent urination, blood in the urine, and straining to urinate. They may also have loss of appetite, lethargy, weakness, vomiting and pain. Urinary stones in the bladder can cause urinary tract infections or more seriously, blockage of the Urethra. Both male and female dogs can be affected, but obstruction of urine flow is more common in males due to differences in anatomy. Although an x-ray can be used to exclude other types of stones, urate stones cannot typically be seen using x-rays and must be evaluated by ultrasound. Not all dogs with mutations in both copies of the SLC2A9 gene will have symptoms of disease, though they will have increased uric acid excretion in the urine.
*All shared disease descriptions are from Pawprint Genetics.
DISEASES OF PRIMARY CONCERN
[ MDR1 ] MULTI-DRUG RESISTANCE MUTATION
Multidrug resistance 1, also called MDR1, is an inherited condition affecting several breeds of dogs, especially herding dogs such as the Scottish collie. The Mutation in the ABCB1 gene associated with MDR1 causes dysfunction of P-glycoprotein, which is responsible for removing certain drugs and toxins from the body. Clinical signs are most commonly associated with distribution of the drug in the central nervous system. MDR1 is inherited in an autosomal incomplete dominant manner in dogs meaning that dogs only need to inherit one copy of the mutated gene to be at an increased risk of developing adverse reactions to certain medications. Though adverse reactions to certain drugs are most commonly seen in dogs having two copies of the mutated gene, Carrier dogs can also experience drug sensitivities and dosages need to be adjusted accordingly. Thus, dogs that have one or two mutant copies of the gene are considered at-risk for adverse drug reactions. If an at-risk dog is treated with one of several common drugs (see below*), they are at risk of developing neurologic symptoms that could range from tremors, excess salivation, anorexia, and blindness to coma and even death. Because of the defective ability to metabolize specific drugs, these drugs can be lethal even at low doses. The MDR1 mutation does not cause adverse effects in dogs unless the dog is exposed to these drugs. Therefore, veterinarians should be notified when a dog is at risk for multidrug resistance 1 prior to administration of any medications.
*Drugs known to cause neurological signs related to the MDR1 mutation:
Acepromazine, butorphanol, doxorubicin, emodepside, erythromycin, ivermectin, loperamide, milbemycin, moxidectin, rifampin, selamectin, vinblastine and vincristine.
In addition to this list, there are many other drugs known to be removed from the central nervous system via the P-glycoprotein mechanism in humans. However, reports of neurological dysfunction related to drugs other than those listed here are scarce in dogs. Please consult your veterinarian prior to giving drugs to known multidrug resistance carriers, affected dogs, or untested dogs of breeds commonly affected with this condition.
[ CEA ] COLLIE EYE ANOMALY
Collie Eye Anomaly (CEA), also known as choroidal hypoplasia (CH), is an inherited disease affecting several dog breeds including the Scottish collie. The choroid is the layer of tissue in the eye responsible for supplying blood and nutrients to the Retina. In dogs affected with CEA, the choroid does not develop properly and is therefore thinner than normal. The severity of the condition can vary from dog to dog. In mild cases, affected dogs may only show signs of collie eye anomaly on eye exam between about 5 and 12 weeks of age, just prior to normal, age-related pigmentation of the retina which often masks the characteristic, disease-related changes. After this time period, mildly affected dogs may be impossible to distinguish from normal dogs on eye exam (a phenomenon often referred to as “going normal”) and may not display obvious vision deficits. In more severely affected dogs, clinical signs include malformations of the eye and/or optic nerve (colobomas), retinal detachment, intraocular bleeding, and subsequent blindness. Both mild and severe forms of CEA are associated with the same NHEJ1gene Mutation. Therefore, predicting the potential severity of the disease in an affected puppy is difficult as mildly affected parents may produce offspring that are severely affected.
DISEASES OF SECONDARY CONCERN
[ CN ] CYCLIC NEUTROPENIA
Cyclic neutropenia is an inherited disease affecting Scottish collies. Affected dogs undergo an oscillating cycle in the quantity of a type of white blood cells called neutrophils, important for controlling and preventing bacterial and fungal infections. Affected dogs have Neutrophil counts oscillating between normal quantities to almost zero neutrophils on an approximate 2 week frequency. Affected puppies often die within a few days of birth or are stunted in growth. Affected dogs have a gray coat color and are vulnerable to infections during periods of low neutrophil counts. Symptoms associated with this condition are normally seen during or immediately after a period of low neutrophil counts. Symptoms include fever, diarrhea, inflamed lymph nodes, gingivitis, lameness and mild bleeding episodes. Even with medical care, most dogs die before 2 years of age due to liver or kidney failure.
[ DM ] DEGENERATIVE MYELOPATHY
Degenerative Myelopathy caused by Mutation of the SOD1 gene is an inherited neurologic disorder of dogs. This mutation is found in many breeds of dog, including the Scottish collie. While it is not clear for some of the other breeds, collies are known to develop degenerative myelopathy associated with this mutation. The variable presentation between breeds suggests that there are environmental or other genetic factors responsible for modifying disease expression. The average age of onset for dogs with degenerative myelopathy is approximately nine years of age. The disease affects the White Matter tissue of the spinal cord and is considered the canine equivalent to amyotrophic lateral sclerosis (Lou Gehrig’s disease) found in humans. Affected dogs usually present in adulthood with gradual muscle Atrophy and loss of coordination typically beginning in the hind limbs due to degeneration of the nerves. The condition is not typically painful for the dog, but will progress until the dog is no longer able to walk. The gait of dogs affected with degenerative myelopathy can be difficult to distinguish from the gait of dogs with hip dysplasia, arthritis of other joints of the hind limbs, or intervertebral disc disease. Late in the progression of disease, dogs may lose fecal and urinary continence and the forelimbs may be affected. Affected dogs may fully lose the ability to walk 6 months to 2 years after the onset of symptoms. Affected medium to large breed dogs, such as the rough collie, can be difficult to manage and owners often elect euthanasia when their dog can no longer support weight in the hind limbs.
[ PRA/PRCD ] PROGRESSIVE RETINAL ATROPHY / PROGRESSIVE ROD-CONE DISEASE
Genetic testing of the RD3 gene will reliably determine whether a dog is a genetic Carrier of progressive retinal Atrophy, Rod-cone dysplasia 2. Progressive retinal atrophy, rod-cone dysplasia 2 is inherited in an Autosomal Recessive manner in dogs meaning that they must receive two copies of the mutated gene (one from each parent) to develop the disease. In general, carrier dogs do not have features of the disease but when bred with another carrier of the same Mutation, there is a risk of having affected pups. Each pup that is born to this pairing has a 25% chance of inheriting the disease and a 50% chance of being a carrier of the RD3 gene mutation. Reliable genetic testing is important for determining breeding practices. In order to eliminate this mutation from breeding lines and to avoid the potential of producing affected pups, breeding of known carriers to each other is not recommended. Dogs that are not carriers of the mutation have no increased risk of having affected pups. However, because there are multiple types of PRA caused by mutations in other genes, a normal result in RD3does not exclude PRA in a pedigree.
[ VWD II ] VON WILLEBRAND'S DISEASE (TYPE II)
VWDII is an inherited blood-clotting disease. Symptoms can include easy bruising, frequent nosebleeds, lots of bleeding from teething, and excessive bleeding from surgery or trauma. When dogs are known VWD-Affected, surgeons can be ready ahead of time with extra blood units on hand, but can be caught off guard when it's not known that a patient is Affected. VWD could also be a problem for simple everyday concerns such as nail trims, but it's especially important for dogs who work bigger stock such as swine or cattle.
[ HUU ] Hyperuricosuria
Hyperuricosuria is an inherited condition of the urinary system affecting several breeds of dog. The SLC2A9 gene codes for a protein that allows the kidneys to transport uric acid from the urine. Dogs with mutations in both copies of the SLC2A9 gene are predisposed to have elevated levels of uric acid in the urine, hence the name hyperuricosuria. Uric acid can form crystals and/or stones (uroliths) in the urinary tract. Dogs with hyperuricosuria most commonly present with symptoms of recurrent urinary tract inflammation, which include frequent urination, blood in the urine, and straining to urinate. They may also have loss of appetite, lethargy, weakness, vomiting and pain. Urinary stones in the bladder can cause urinary tract infections or more seriously, blockage of the Urethra. Both male and female dogs can be affected, but obstruction of urine flow is more common in males due to differences in anatomy. Although an x-ray can be used to exclude other types of stones, urate stones cannot typically be seen using x-rays and must be evaluated by ultrasound. Not all dogs with mutations in both copies of the SLC2A9 gene will have symptoms of disease, though they will have increased uric acid excretion in the urine.
*All shared disease descriptions are from Pawprint Genetics.